
 Agilent 6890N ECD.
Agilent 6890N ECD.Temp Ramp: 80℃(1min)----20℃/min--- 260℃(15min)----5℃/min---300℃(3min),
Detector Temp: 300℃
Inlet: 250℃
GC Column:HP-5.
 (1) Maybe the collision energy is too LOW. The First MS is not full a fragment.
(1) Maybe the collision energy is too LOW. The First MS is not full a fragment.
 The molecular weight of your analyte is 548: 1st Mass is 549, 2nd Mass is 489, 3rd Mass are 471, 425.
The molecular weight of your analyte is 548: 1st Mass is 549, 2nd Mass is 489, 3rd Mass are 471, 425. Come on, Man: Don't be silly to send us this Newbie question? Are you want to test our GC knowledge?
Come on, Man: Don't be silly to send us this Newbie question? Are you want to test our GC knowledge?
 (1) From the reviewer's point, you should explain why the Low response in the Mass Spectrum?
(1) From the reviewer's point, you should explain why the Low response in the Mass Spectrum? Which detergent used in the Lab now? Look like the contamination of the detergent/surfactant which was used for the glassware.
Which detergent used in the Lab now? Look like the contamination of the detergent/surfactant which was used for the glassware. That is really a good example for Triple Quadrupole LC-MS.
That is really a good example for Triple Quadrupole LC-MS.So we always could change pH value to alter the disassociation of analyte in ESI mode. ( With ESI, convert the molecular ion from liquid phase into gas phase, enable the analyte evaporate into ion status -> obtain a decent response).
From the pKa, we know about the nature of analyte: basic,acid or neutral.
Then for basic analyte -> add some Acetic Acid/ Formic Acid ( pH ≈ pKa-2 ) -> enable the analyte generate positive (+) ion in solution for Positive ESI (+) mode. or for acid analyte -> add some ammonium to increase the pH ≈ pKa+2 -> analyte is molecular ion with negative (-) for Negative ESI (-) mode.
Next time, will update my Tech Notebook about how to trouble-shooting the Poor Response of the Analyte based on the API 3200 Tandem QQQ LC-MS . (that is a golden treasure I learnd from Tech, U definetely need to buy me a drink for that)
 Talked about this issue so many time, need to assume that response factor of each component is identical. However, we always negelect that at the assay of total impurities in the samples were performed by normalization method.
Talked about this issue so many time, need to assume that response factor of each component is identical. However, we always negelect that at the assay of total impurities in the samples were performed by normalization method. So I only recommend to use this method to estimate the relative amounts of small impurities or degradation compounds in a purified component at the specific detector wavelength, that is chromatographic purity.
So I only recommend to use this method to estimate the relative amounts of small impurities or degradation compounds in a purified component at the specific detector wavelength, that is chromatographic purity. From my view, the Second maybe ensure a better Repeatability for your results.
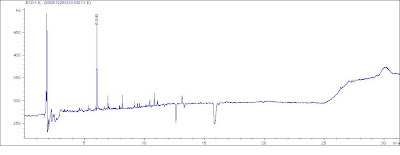
From my view, the Second maybe ensure a better Repeatability for your results.  The peak @ rt=2.8 is the problem?
The peak @ rt=2.8 is the problem?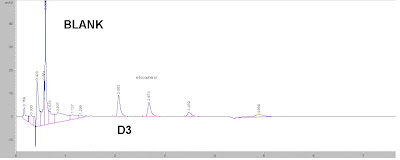 (1) Double check the contamination from the needles and HPLC volumetric flash, vials.
(1) Double check the contamination from the needles and HPLC volumetric flash, vials.  Here are my suggestions:
Here are my suggestions: At the on-site setup@Feb-2007, already learned how to tune up the 6410. At March 2007, Mr. B-Cousin** just occasional stopped by Atlanta: then asked him for an update of Mass-Hunter Software. Then I performed an auto-tune on the 6410. After that, the nightmare on the Negative ESI happened. -> Found poor selectivity on the Negative ESI mode ->However the Positive ESI mode worked perfect with a good pass results of tuning. Tried to cal Agilent Tech, but faile to find out a solution.
At the on-site setup@Feb-2007, already learned how to tune up the 6410. At March 2007, Mr. B-Cousin** just occasional stopped by Atlanta: then asked him for an update of Mass-Hunter Software. Then I performed an auto-tune on the 6410. After that, the nightmare on the Negative ESI happened. -> Found poor selectivity on the Negative ESI mode ->However the Positive ESI mode worked perfect with a good pass results of tuning. Tried to cal Agilent Tech, but faile to find out a solution. When cool down, checked with the old tune reports with new tune reports: for old tuning solution b4 upgrade the 6410 Masshunter software, tuning peaks@ 112.99, 431.98, 601.98, 1033.99, 1633.95, 2233.91. However when upgrade the software, the tuning m/z is: 112.99, 302.00, 601.98, 1033.99, 1333.97, 1633.95.
When cool down, checked with the old tune reports with new tune reports: for old tuning solution b4 upgrade the 6410 Masshunter software, tuning peaks@ 112.99, 431.98, 601.98, 1033.99, 1633.95, 2233.91. However when upgrade the software, the tuning m/z is: 112.99, 302.00, 601.98, 1033.99, 1333.97, 1633.95. So when the m/z of the analyte is from 230- 350 at NegESI, almost got poor results, made the catalyst guys crazy and cry everyday.
So when the m/z of the analyte is from 230- 350 at NegESI, almost got poor results, made the catalyst guys crazy and cry everyday.
 To my best knowledge, this analyte (Dimethyl 1,3-acetonedicarboxylate, cas#1830-54-2) should not be a problem for you.
To my best knowledge, this analyte (Dimethyl 1,3-acetonedicarboxylate, cas#1830-54-2) should not be a problem for you. If the assay method is well developed and transferred, so my first comment to your question is the bleeding of the Capillary Columns. ( Column bleed is a result of stationary phase fragments releasing from the inside wall of the column. )
If the assay method is well developed and transferred, so my first comment to your question is the bleeding of the Capillary Columns. ( Column bleed is a result of stationary phase fragments releasing from the inside wall of the column. ) Here are some comments:
Here are some comments: Peak @ 21.36 min -> 2,6-di-tert-butyl-4-sec-butylphenol
Peak @ 21.36 min -> 2,6-di-tert-butyl-4-sec-butylphenolBeing an Integrity man.