skip to main |
skip to sidebar
 (1) Capillary Voltage: -3.0 kv.(2) Cone Voltage: -40 v.(3) Collision Energy: 18 ev.(4) MRM: m/z 299.5 →m/z 255.2.
(1) Capillary Voltage: -3.0 kv.(2) Cone Voltage: -40 v.(3) Collision Energy: 18 ev.(4) MRM: m/z 299.5 →m/z 255.2.

 Happy Spring Festivals 2009!
Happy Spring Festivals 2009!
(1) Compounds That Cause Sensitivity Suppression.
-Salts can interfere with ionization and can cluster to complicate spectrum (but also aid in identification).
-Strong bases or quaternary amines can interfere with positive mode analytes, e.g. Triethylamine(TFA).
-Acid -Sulfuric/Sulfonicacids and TFA interfere in negative mode.
-Phosphate buffer and non-volatile ion pairing agents (e.g. SDS) can cause severe suppression and complex spectrum.
(2) Dimerization([2M+H]+) can occur at high concentration, leading to non-linearity during quantitation.
-DimerSignal at m/z = (MW*2) + 1.
-Can cause non-linearity at high concentrations.
The current global shortage of Acetonitrile has pushed us to find 5 ways to reduce thedependence on AcetonitrileOption 1: Reduce column ID:
Reducing the HPLC column ID from 4.6 mm to 3.0 mm can result in > 50 % reduction in mobile phase consumption. Option 2: Reduce particle size and column dimensions: * Reduces solvent consumption * Maintains resolution * Reduces run timeOption 3: Reduce column length:Option 4: Substitute Acetonitrile with Methanol:http://www.phenomenex.com/images/methanal_replacement.gifOption 5: Solvent recycling (Isocratic separations only)Reference by Phenomenex.

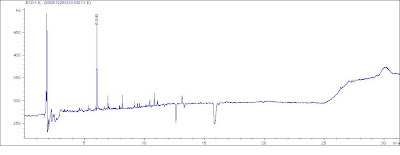 Agilent 6890N ECD.Temp Ramp: 80℃(1min)----20℃/min--- 260℃(15min)----5℃/min---300℃(3min),Detector Temp: 300℃Inlet: 250℃GC Column:HP-5.
Agilent 6890N ECD.Temp Ramp: 80℃(1min)----20℃/min--- 260℃(15min)----5℃/min---300℃(3min),Detector Temp: 300℃Inlet: 250℃GC Column:HP-5.
 (1) Maybe the collision energy is too LOW. The First MS is not full a fragment.(2) Are you using ESI mode?(3) If the Molecular weight is 237, for SIM -> Here are three characteristic ions (m/z) 164,149,122 I highly recommend them.(4) MS-MS (MRM): Need to search online for the product ion and parent ions.(5) If only need the full scan, why not adjust the concentration of analyte.Hope it helps.
(1) Maybe the collision energy is too LOW. The First MS is not full a fragment.(2) Are you using ESI mode?(3) If the Molecular weight is 237, for SIM -> Here are three characteristic ions (m/z) 164,149,122 I highly recommend them.(4) MS-MS (MRM): Need to search online for the product ion and parent ions.(5) If only need the full scan, why not adjust the concentration of analyte.Hope it helps.


 (1) Maybe the collision energy is too LOW. The First MS is not full a fragment.
(1) Maybe the collision energy is too LOW. The First MS is not full a fragment.